09.06.20
Bleepa® becomes the first UK CE marked communication platform
Words provided by Feedback Medical, editorially reviewed by National Health Executive
Feedback Medical, the specialist medical imaging technology company, has announced, in compliance with the Medical Device Directive (MDD) and having met the stringent criteria associated with the manufacture of a medical device, it has affixed a CE Mark to Bleepa, confirming its use as a Class 1 Medical Device.
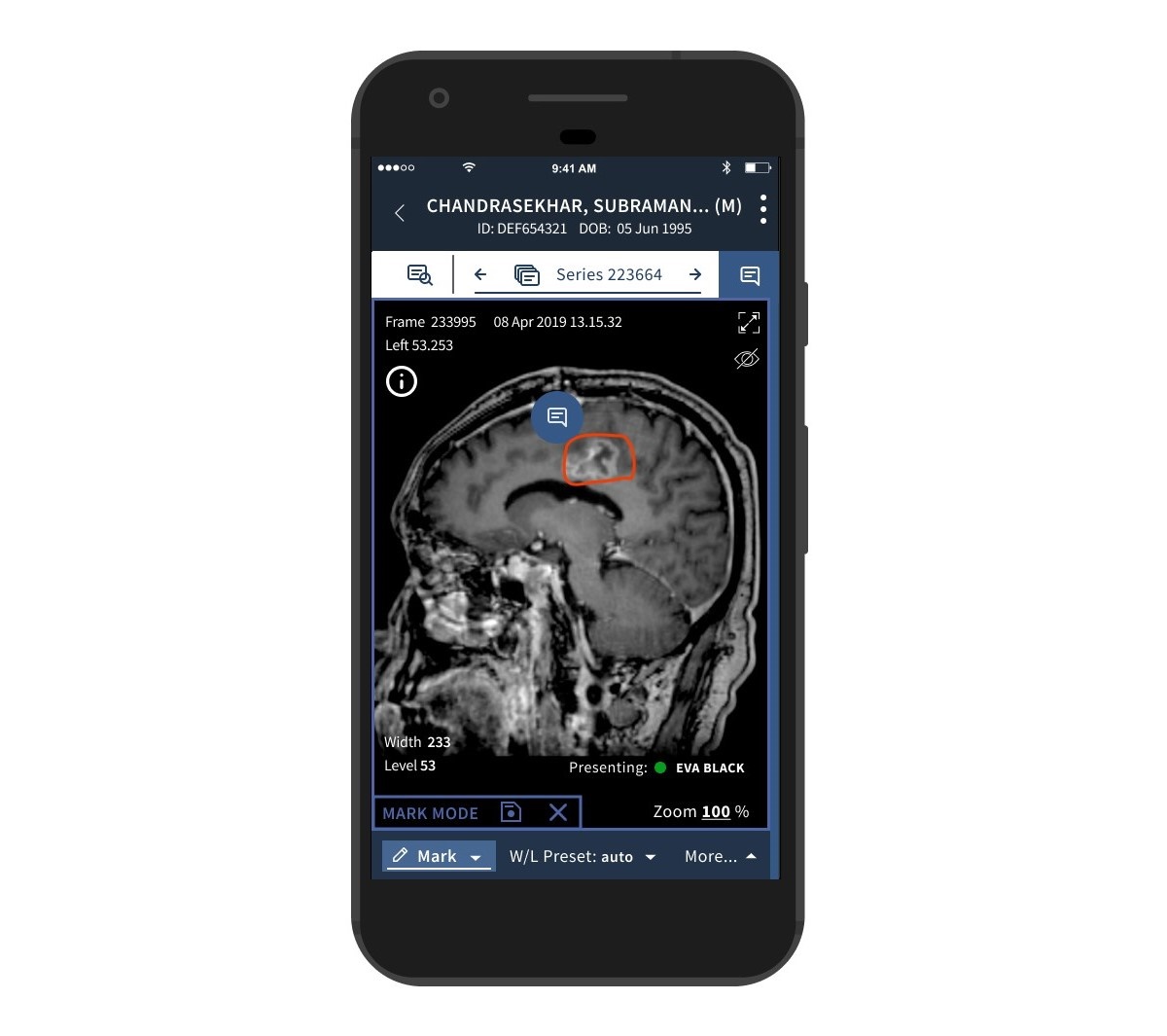
Bleepa is Feedback Medical’s flagship imaging-based communications platform for clinicians to access medical grade images through smartphones, tablets and desktops.
It becomes the first product of its kind to receive the accreditation, bringing significant benefits to clinicians who use the products and the patients they serve. For the first time, clinicians can, in one place, access through smartphones, tablets and desktops rapid clinical messaging and review of medical grade imaging for all members of a clinical team, directly from a hospital Picture Archiving and Communications System (PACS).
Bleepa provides instant and remote access to clinical grade medical images through a zero footprint application. The majority of patient cases require medical imaging which can be seamlessly shared to the entire clinical team through Bleepa. Cases can be discussed through its secure instant messaging and image annotation, allowing comments and treatment decisions to be communicated instantly between team members.
CEO of Feedback Medical, Dr Tom Oakley, said: “We believe that Bleepa is the future for communicating about patient care and is already changing the way in which clinicians discuss cases and process patients through the hospital system. The CE mark is an important milestone in its commercialisation and sets Feedback apart from other providers.
“Pennine Acute Hospitals NHS Trust is already demonstrating the value of using Bleepa across the hospital setting. This important regulatory milestone offers healthcare providers confidence in our product over other unregulated providers as we continue the roll-out of Bleepa in support of the NHS, both to fight against COVID-19 as well in day-to-day patient management.”
Bleepa is Feedback Medical’s flagship imaging-based communications platform for clinicians to access medical grade images through smartphones, tablets and desktops
Clinicians and hospitals are advised by the MHRA to use medical devices with a CE Mark, representing the device has met the legal requirements for safety, quality and performance when it is used as the manufacturer instructs. The MHRA further advises that off-label use of a device, such as use of a device that does not carry an appropriate CE Mark, will be at such user’s own risk and such person or their employer could become liable for civil claims for damages from injured patients or their families if something goes wrong with the device.
As a result of this, having the CE Mark is a major step forward for the Bleepa product and makes it unique to the market.
Annex 1: Illustrative examples of qualification for software used in the healthcare environment c) Information Systems: "The modules used with electronic patient record system modules that might be qualified in their own right as medical devices are for example: - an image viewer with functionality for diagnosis based on digital images; - a medication module."
Dr Oakley added: “We believe that many NHS sites are unknowingly using uncertified tools and are therefore exposed to civil claims arising from use of those tools. Bleepa will enable Trusts to engage the digital communication revolution safely and securely within a regulated environment.
“Ensuring the roll out of a regulated communication platform should be a key priority for the NHS in its efforts to combat COVID-19 and beyond. Bleepa is now uniquely positioned to deliver against this need.”
An essential part of CE accreditation is real world feedback and the deployment of Bleepa at Pennine Hospitals NHS Trust not only generated the required clinical feedback but led to additional improvements in the product which have now been incorporated into the CE-marked version of Bleepa.
This CE-marked version of Bleepa will now be deployed at other NHS sites.