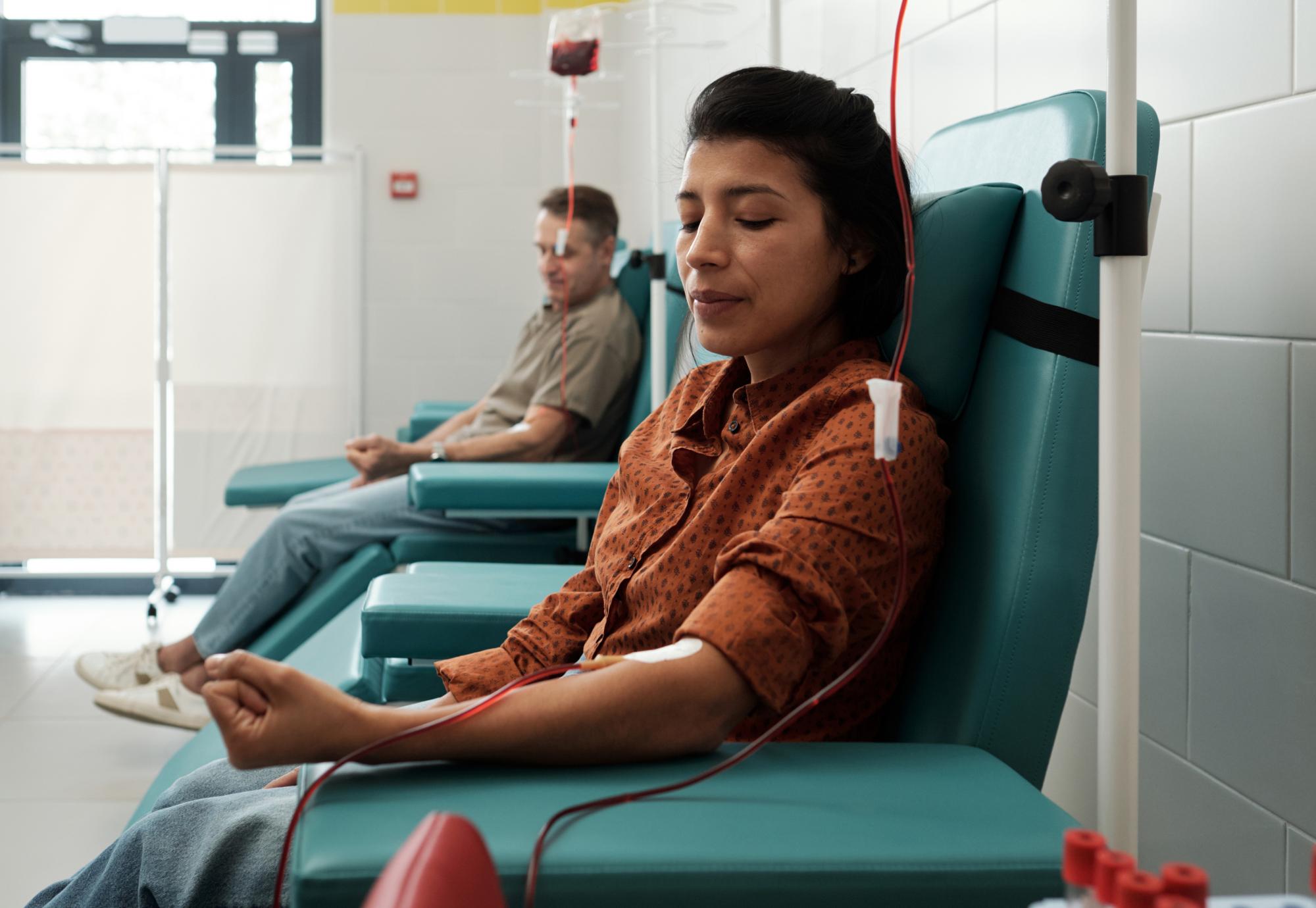
NHS England is offering stem cell transplants to patients with thalassaemia, to cure their condition and help avoid blood transfusions for the rest of their lives.
Thalassaemia impacts the production of haemoglobin in the blood, with severe anaemia and tiredness to patients, whilst also forcing sufferers to undergo blood transfusions every two to four weeks to survive. Now, however, the NHS is able to offer curative stem cell transplants for adults with thalassaemia, with the treatment funded by the health service.
NHS England has estimated that there are over 600 adults across the United Kingdom who are relying on transfusions to deal with thalassaemia and are eligible for new treatment.
The process, named allogeneic haematopoietic stem cell transplant (Allo-HSCT), will see a patient’s bone marrow stem cells replaced by those from a donor, with the cells being given via an intravenous infusion which will support the establishment of healthy blood cell reproduction. This is the first time that adults will be offered the treatment, with it previously only offered to children due to the complication risks for adults.
Thanks to new advances in the treatment of patients before, during and after transplants, patients over the age of 18 are now able to be recommended the treatment, supported by new guidance from the Clinical Priorities Advisory Group.

Medical Director at NHS England, Professor Sir Stephen Powis, said:
“Expanding the availability of stem cell transplants to adults living with thalassaemia is another vital step forward to help change the lives of those living with this deeply debilitating condition.
“Thalassaemia can be an incredibly painful condition with difficult symptoms for patients as well as the impact on their heart, liver and bones, and it’s fantastic that offering this evidence-based curative stem cell treatment can now offer new hope to help significantly improve patients’ quality of life.”
Health inequalities across the country are also being tackled through this treatment, with thalassaemia being more prevalent among members of the Southern European, Middle Eastern, South American, Caribbean, Asian, and South East Asian communities.
Romaine Maharaj, UK Thalassaemia Society’s Executive Director, also commented:
“We celebrate the long-awaited approval of Allo-HSCT for adults with transfusion-dependent thalassaemia. This remarkable milestone offers hope to adults with donor matches who were previously excluded from accessing a curative option.
“While it is a huge step in the right direction and a monumental win for thalassaemia, we also eagerly await the much-needed approval for gene therapies. Having both curative options available will grant more patients the chance to live transfusion-independent lives, enhancing both their quality of life and life expectancy.”
Image credit: iStock