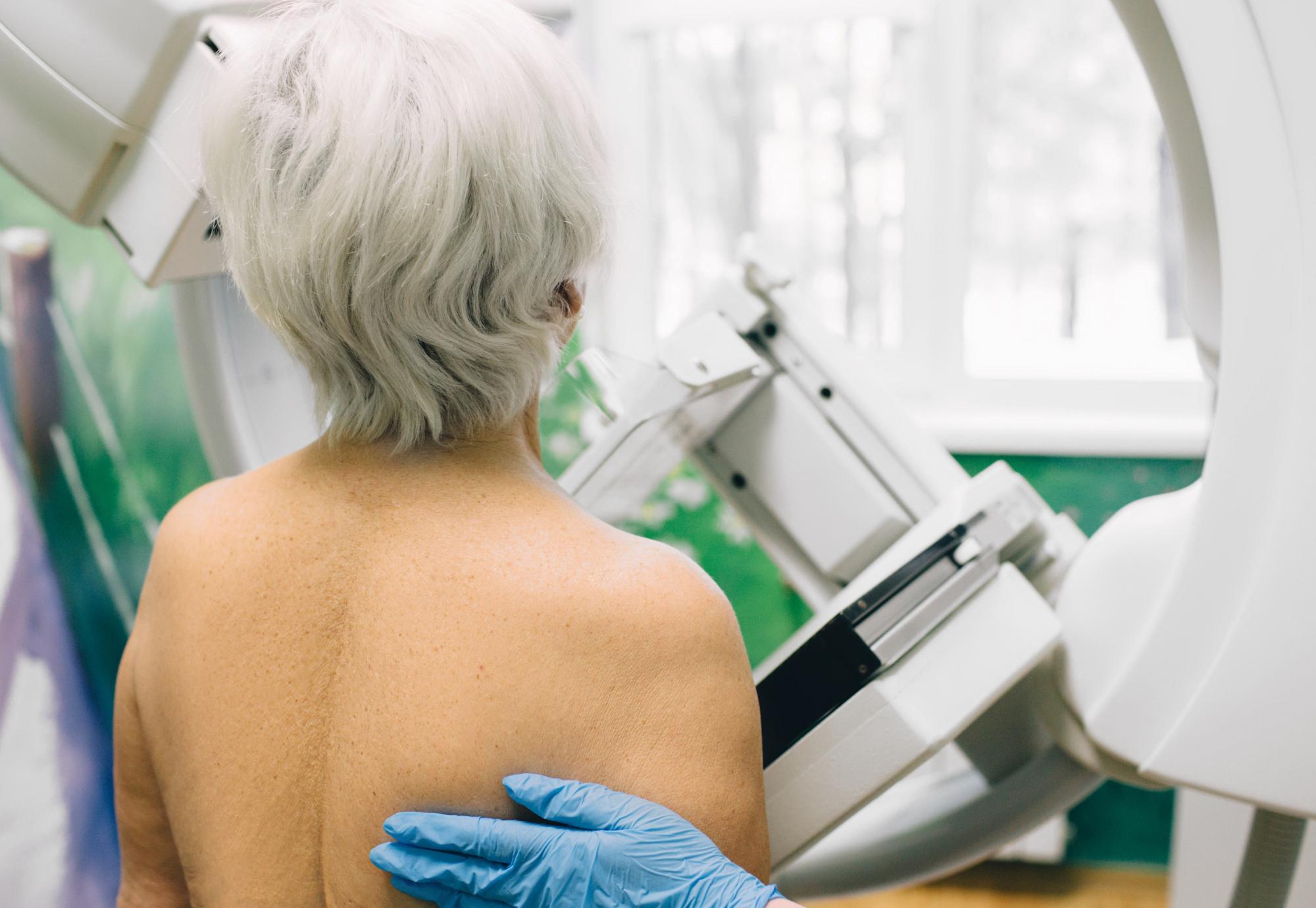
The National Institute for Health and Care Excellence (NICE) has issued final draft guidance recommending elacestrant (KORSERDU), a new once-a-day tablet, for treating a specific type of advanced breast cancer.
Set to benefit approximately 1,100 patients in the UK, Elacestrant is recommended for patients with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative (HER2-), locally advanced or metastatic breast cancer that has an activating ESR1 mutation. This recommendation applies to patients whose cancer has progressed after at least 12 months of hormone therapy and treatment with a CDK 4 and 6 inhibitor.
This latest guidance marks a significant milestone, as it means NICE has now recommended 22 out of the 23 breast cancer treatments it has assessed over the past six years. Until now, there have been no targeted treatments available on the NHS for advanced breast cancer with an ESR1 mutation.
Elacestrant can also be used for patients whose cancer has mutations in both the PIK3CA and ESR1 genes. Initially, NICE did not recommend elacestrant due to uncertainties regarding the most appropriate comparator and the evidence of its effectiveness compared to standard care. However, after working closely with the manufacturer to address these issues, the independent appraisal committee decided that a severity weighting of 1.2 could be applied to patients with an activating ESR1 mutation.

Commenting on the decision, Director of Medicines Evaluation at NICE, Helen Knight, said:
“The committee heard from the clinical and patient experts that people whose advanced breast cancer has an ESR1 mutation tend to have worse survival than people whose cancer doesn’t have this mutation. Disease progression also tends to be faster for breast cancer with an ESR1 mutation.
“The committee understood that patients living with advanced breast cancer prioritise treatments that extend life, support quality of life, and delay the need for chemotherapy, while being safe and tolerable.
“Elacestrant is a promising new treatment with the potential to address these priorities. We’re therefore pleased to be able to recommend it as a good use of NHS resources and value for money for taxpayers.”
By considering the severity of the disease, NICE concluded that elacestrant is a cost-effective use of NHS resources for patients with ESR1 mutations, as well as those with both ESR1 and PIK3CA mutations. This decision underscores NICE's commitment to providing innovative and effective treatments for patients with advanced breast cancer.
NICE's recommendation of elacestrant represents a significant advancement in the treatment of advanced breast cancer, offering new hope to patients with limited options.
Image credit: iStock