On the same day the Medicines and Healthcare products Regulatory Agency (MHRA) granted it a licence, the National Institute for Health and Care Excellence (NICE) has recommended etrasimod as a new treatment for ulcerative colitis.
Etrasimod, which is marketed as Velsipity by Pfizer, has been shown to be more effective than placebos in clinical trials and is expected to benefit 25,000 people in England.
It is likely to be more effective than the immunotherapy known as adalimumab too, according to NICE after indirect comparisons.
Etrasimod has therefore been recommended by NICE for those aged 16 and over with moderate to severe ulcerative colitis, who cannot have conventional therapy or biological treatment.
“…a positive difference for thousands of people.”
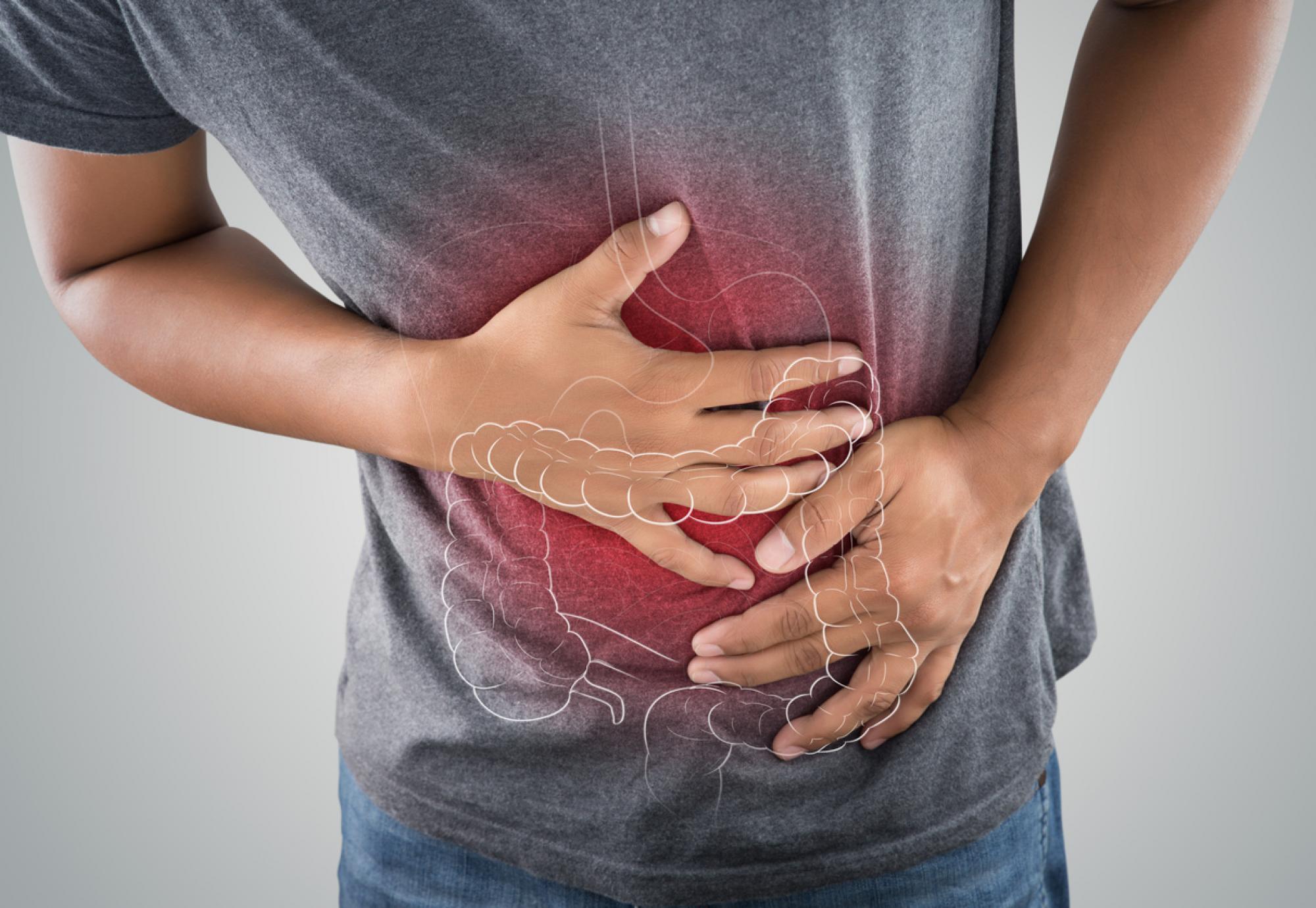
Ulcerative colitis is a long-term disease where the colon and rectum become inflamed which can cause ulcers to develop, potentially leading to diarrhoea, arthritis and osteoporosis.
Approximately 300,000 people in the UK have been diagnosed with the disease, with diagnosis primarily occurring in 15-25-year-olds although it can develop at any time.
“Severe ulcerative colitis is a debilitating lifelong condition; etrasimod provides a new convenient and effective treatment option that will make a positive difference for thousands of people,” said Helen Knight, NICE’s director of medicines evaluation.
Helen added: “I’m very pleased we have been able to publish our final guidance recommending the treatment on the day the MHRA granted it a licence. We are determined to continue getting the best care to patients fast.”
A confidential commercial arrangement will make the treatment available to the NHS at a discount.
The most common side effects of the treatment include bradycardia, hypertension, urinary tract infection, and lower respiratory tract infection.
Image credit: iStock