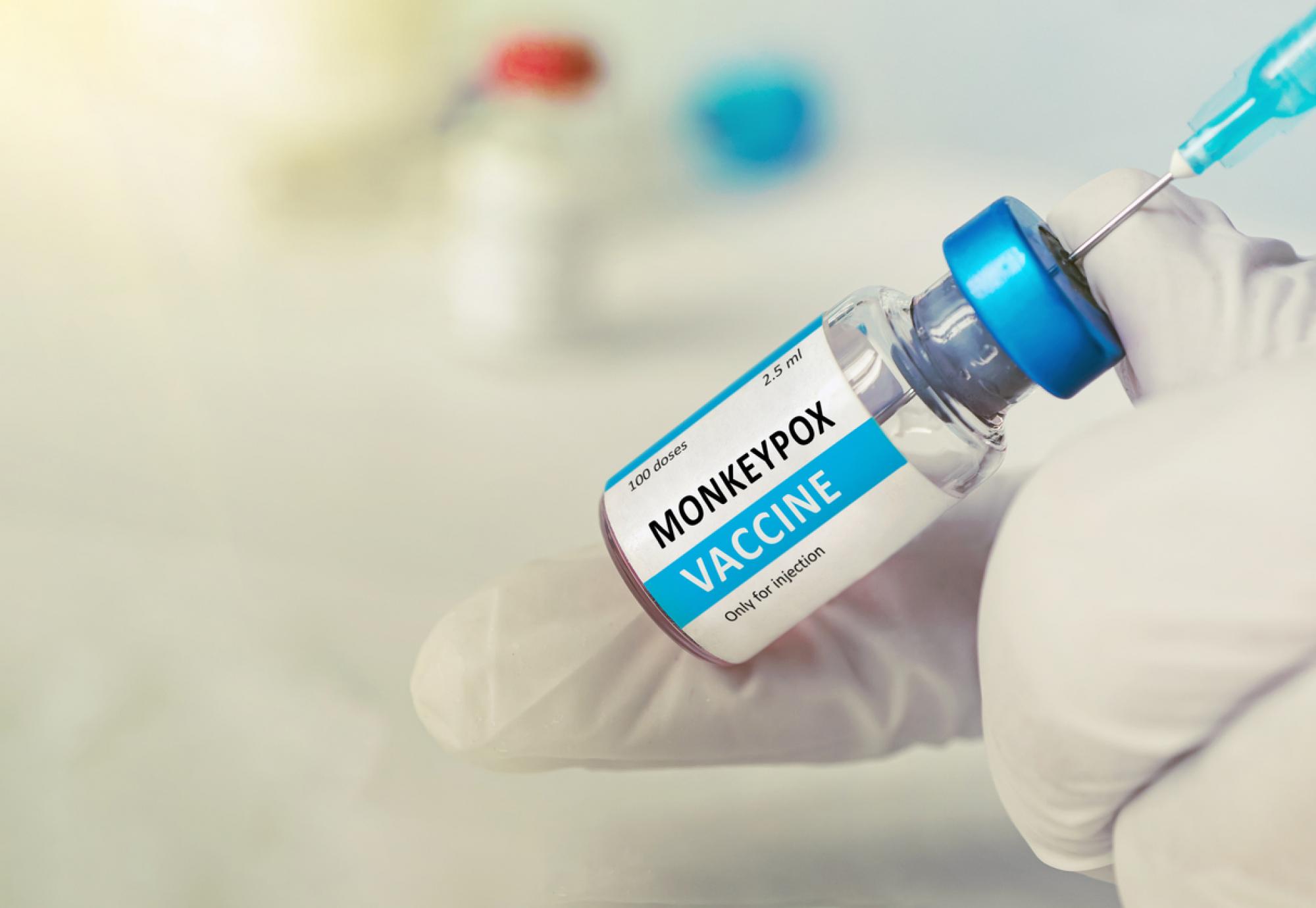
The NHS are set to trial giving patients smaller doses of the monkeypox vaccine in a bid to optimise the reach of supplies and protect more people from the virus.
The innovative method, referred to as ‘fractional dosing’, will entail administering a smaller, but equally effective, dose of the monkeypox vaccine to patients, in an approach that the Government say could quintuple the amount of people insulated against the virus.
Results from clinical trials indicated that the fractional dosing method provided an almost identical immune response in patients, and now, following suit of the US, the UK are set to pilot the strategy in three NHS sites.
Dr Mary Ramsay, Head of Immunisation at UKHSA, said: “Global supplies of the smallpox vaccine used to combat monkeypox are limited but we acted early to ensure the UK obtained the maximum number of doses available.
“Adopting this tried and tested technique will help to maximise the reach of our remaining stock, including the 100,000 doses due to arrive in the country next month, potentially enabling us to offer protection for many more thousands of people.
“We will continue to remain agile in our response to the monkeypox outbreak and will adapt our approach as new science and advice becomes available.”
Following approval from the European Medicines Agency Emergency Task Force, the UK Health Security Agency (UKHSA) and the Joint Committee on Vaccination and Immunisation (JCVI) have now reviewed evidence of the fractional dosing method’s benefits.
As a result of their deliberations, the UKHSA and JCVI are now working alongside NHS England to give patients at Chelsea and Westminster NHS Foundation Trust, Central and North West London NHS Foundation Trust and Locala Health and Wellbeing in Greater Manchester a 0.1ml dose of the monkeypox vaccine rather the standard 0.5ml.
Professor Sir Andrew Pollard, Chair of the JCVI, said: “The use of fractional dosing will allow more people to be vaccinated sooner by optimising use of the constrained vaccine supply, and this approach is expected to reduce the spread of monkeypox.
“Dosing in this way has been successfully used in outbreaks of other viral diseases around the world and existing data we have reviewed indicates this should not compromise protection.”
As well as trialling the reduced dosing, the UKHSA have decided that, as a result of finite vaccine supplies, the post-exposure offer of vaccines should be limited to those who are at the most risk of serious illness – an approach that has been co-signed by the JCVI.
This will not change the terms of the pre-exposure offer of the vaccine, just that, once infected, vaccines will be reserved for people with immunosuppression, pregnant women and children under the age of 5.
More information on the trial is available here.